The medical electronics market is massive, both globally and in the U.S., and is expanding and evolving rapidly, fueled by growth in both traditional segments, like diagnostics equipment, and newer, more cutting-edge segments, like portable and wearable networked devices. But despite the diversity of the expansive medical electronics market, two things are always true: Medical electronics require a safe, reliable source of power, and they must adhere to stringent safety regulations. To learn more about powering medical electronics, we spoke with MEAN WELL USA Product Manager Kai Li.
The medical electronics market is a vital, expansive, and rapidly evolving market. According to a recent Precedence Research report, it was worth $85 billion globally in 2022 and $22.68 billion here in the U.S. The largest product category by market share was therapeutic devices, which include medical implantable devices, respiratory care devices, and surgical robots, and the largest application area was hospitals. By 2032 the medical electronics market is expected to grow to a value of $253.57 billion globally and $67.65 billion in the U.S., largely fueled by remarkable growth in the diagnostics segment, which includes ultrasound, X-ray, and MRI equipment, and the home healthcare segment, which is driving demand for and innovation in portable, wearable, and networked medical electronics.
Medical electronics have long provided solutions for accurate diagnostics, continuous monitoring, and effective treatment, and as they’ve evolved, they’ve also come to provide better patient outcomes, reduced hospitalization, and improved overall health. Today, the massive medical electronics market extends from miniature implantable devices, like pacemakers, to wearable devices, like ECG monitors; handheld devices, like pulse oximeters; portable devices, like therapeutic ultrasound machines; and equipment that still typically takes up the better part of a room, like MRI scanners. But despite the diversity of the expansive medical electronics market, two things are always true. One, medical electronics, like all electronics, require a safe, reliable source of power, and two, they must adhere to an array of complex and rigorous quality and safety regulations that are notorious for extending medical OEM design cycles and driving up development costs.

To learn more about powering medical electronics, we spoke with MEAN WELL USA Product Manager Kai Li, who addressed market-driven and regulatory design demands, shared some specification tips, and introduced MEAN WELL’s unique value as well as several different types of medical power supplies and the types of applications they’re ideally suited for.
Hi Kai. Please introduce yourself.
Hi. I’m Kai Li, and I’m a product manager at MEAN WELL USA. I studied electrical engineering at UCLA, where I had the opportunity to learn from some of the industry’s best and brightest minds and to participate in several valuable enrichment activities, such as collaboratively developing a line-following robotic racecar for IEEE’s NATCAR competition and designing and testing a working laser communication system that can transmit a modulated data signal, like music, over a distance of more than 1,000 feet. I also completed two internships at companies that develop essential equipment for the semiconductor industry.
After graduating, I came to work at MEAN WELL, and come May, I’ll have been here for eight years. I started as an application engineer, working to solve customer application issues with power supplies and evaluating modifications to meet customer’s niche demands, and then got promoted to product manager. Currently, I spend most of my time working on new product development proposals for the North American market and training sales and distributors about new product launches.
Please also introduce us to MEAN WELL.
MEAN WELL was founded in 1982 on good intentions and fair and honest business practices and, over the course of the last 42 years, has established itself as a leading global power supply manufacturer. MEAN WELL is one of just a few global manufacturers dedicated to standard power supply products, and according to a March 2023 Micro Technology report, we’re the third highest-ranked DC-output power supply manufacturer in the world. We’re also ranked eighth in terms of global medical power supply market share.
You joined us today to talk about power supplies for the medical market. What are medical device and equipment manufacturers looking for when it comes to power supplies?
In general, medical device and equipment manufacturers are looking for power supplies with features including broad regulatory compliance, high reliability, electrical stability, safety features (like overvoltage, overcurrent, and overload protection and MOOP operator and MOPP patient electrical shock protection), energy efficiency, low leakage, miniaturization for space and weight savings, and versatility in terms of electrical performance (e.g., voltage, wattage, and operating temperature ratings), configurability, and application suitability.
Those that serve the growing home healthcare and telemedicine markets are also likely to be interested in power supplies with a measure of environmental protection, such as IP20 or IP42 ratings. These markets utilize a lot of peripheral and wearable devices, including heart rate and body index monitors, that usually come with external power adapters for charging. And since the devices themselves are small and portable, OEMs have a strong preference for smaller, lightweight power adapters and designs that support not only local but global portability, such as external power adapters that offer interchangeable AC plug accessories.
While it makes sense given the inherent risks associated with medical devices and equipment, that’s a lot of product characteristics to consider. Do you have any advice for medical manufacturers looking to specify power supplies?
Definitely! First, consider your application area and end product. For example, laboratory equipment, home healthcare devices, and portable medical devices designed for use in clinical settings will all have different requirements based on the environmental hazards they’re likely to be exposed to. Similarly, different medical equipment and devices will have different requirements for power supply installation. Medical products exposed to more environmental hazards may require built-in enclosed power supplies, those exposed to shock and vibration might require PCB screw-mount power supplies, and those designed for portability might require external power adapters that are easy to install, operate, and replace.
Next, determine whether your end product requires an earth ground connection or not. If so, you’ll need a Class I medical power supply with functional earth grounding, and if not, you’ll want a Class II medical power supply without functional earth grounding, as you only want to pay for the capabilities that you need to help control costs.
Once you’ve got that all sorted, you’ll want to verify your application’s current and voltage requirements, as medical power supplies are available with a wide range of electrical ratings, and identify the typical operating temperature range to ensure that the medical power supply you select can deliver the performance you need.
Lastly, identify any critical specifications that your end product has to meet, such as MOPP or MOOP isolation levels, acceptable leakage current levels, and Class A or B EMC performance. Gathering all this information should make it quicker and easier to identify suitable power supplies, but you can also always contact the manufacturer or an authorized distributor for validation or additional support.
I know that all medical electronics require a power supply of some sort, but can you give us some examples of the types of medical equipment and devices that would need internal versus external power supplies?
Sure. Equipment used for clinical diagnoses, like biochemical and DNA analyzers and enzyme immunoassays, typically require internal power supplies. Diagnostic imaging systems, including X-ray, MRI, and PET scan machines, and ultrasonic instruments, and rehabilitation treatment devices, including cardiopulmonary resuscitation machines, low-frequency therapy devices like TENS units, deep vein thrombosis, and heating pads, can use internal or external power supplies depending on the application area and end product. And home healthcare equipment designed for portability, like vaporizers, nebulizers, blood oxygen meters, and physiotherapy devices, typically require a small and lightweight external medical adapter with a pluggable, replaceable AC socket.
What’s unique about MEAN WELL medical power supplies?
We pride ourselves on being the world’s number one standard power supply manufacturer — an accolade we earned by offering the world’s most comprehensive line of standard, off-the-shelf power supply products — and this dedicated focus, combined with the extensive depth and breadth of our product portfolio, allows us to provide customers in the medical market with unparalleled accessibility. Customers don’t need to come to us with their design needs and then wait for a drawing, a prototype, and various safety approvals. Instead, they can work with us or one of our more than 200 authorized distribution partners, like RS, to identify one of our standard off-the-shelf power supply products that suits their needs.
Our newly expanded portfolio of medical power supplies includes a wide variety of open-frame and enclosed power supplies, DC to DC power modules, and power adapters based on decades of experience and expertise, and all of them already have the industry approvals necessary to ensure compliance with the medical market’s stringent safety standards, which helps reduce design cycle costs and speed up time to market.
Please introduce us to a few of MEAN WELL’s most popular medical power supplies.
Our RPS Series medical-grade, open-frame power supplies are built-in, PCB-type, single-output power supplies based on our proven EPP Series industrial open-frame power supplies. They’re available in common PCB sizes, including 3×2″, 4×2″, and 5×3″, and key features include ultra-low no-load power consumption ranging from less than 0.075W to 0.75W, depending on the model, and a 2xMOPP isolation rating for adequate patient protection. RPS Series power supplies are suitable for BF-type medical applications that come into contact with patients during normal use but don’t touch their hearts, including dental chairs, mobile clinic carts, and electric beds. Unique products in the RPS Series include the RPS-120S and RPS-500.

The RPS-120S is the only medical-grade, 3×2”, PCB-type power supply on the market that can operate up to 120W using free air convection — 20W higher than the maximum power rating achieved by comparable power supplies without external fan cooling. Other features include 150W peak load capability for 10 seconds, 94% efficiency, and rated operating temperatures extending from -30°C to +85°C. It’s also compatible with Class I and Class II installations, which means it can be used in both plastic and metal enclosures and can save system designers time and cost associated with EMI debugging. The RPS-120S is suitable for all types of medical applications that require low leakage current and low standby power consumption, including medical diagnostic equipment, blood analysis instrument, and medical monitoring equipment.
Our new LOP Series open-frame switching power supplies were developed in response to miniaturization and high-density design trends and customer demands for slimmer profiles, higher power capabilities, and a wider operating temperature range. LOP Series PCB-type power supplies deliver high quality, efficiency, and reliability in a cost-effective, low-profile design rated for 12–54V, 600W, and operating temperatures extending from -40°C to +80°C. They also exhibit excellent EMC and safety performance and extremely low leakage and can provide 150% load capacity in just three seconds. The series is suitable for use in Class I and Class II systems and compatible with OVCIII, 2xMOPP, and BF-type medical applications, including a wide range of therapeutic, diagnostic, laboratory, monitoring, and medical robotics applications.
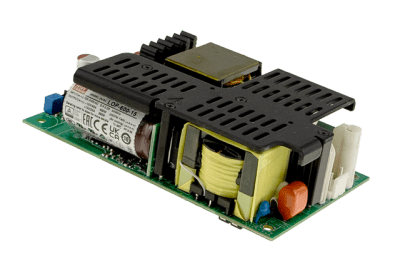
Another key feature of the new LOP Series is that it offers safety certifications for the three different application areas it can be used in — IEC 60601-1 for medical electronics, IEC 62368-1 for consumer electronics, including information technology and telecom equipment, and IEC 60335-1 for household appliances — in addition to IEC 61558-1, which governs the general safety of transformers, reactors, power supply units and is important because not all countries have harmonized to IEC 60601 medical standards. Some still rely on household and transformer safety standards. So, this broad range of IEC compliance makes it easier for OEMs in various industries to serve their global markets with a single SKU.

Our MSP Series medical-grade, enclosed-type power supplies are rated for 100–1,000W, 3.3–48V output voltages, and 2xMOPP/MOOP isolation for adequate operator and patient protection. Additional features include parallel operation capabilities, 5V auxiliary power, remote control functions, including on/off and DC OK signal, and a five-year warranty, and they are ideal for medical applications including imaging, diagnostic, treatment, and laboratory analysis systems.
The MSP-1000 Series is designed to replace our MSP-600 Series power supplies in the same footprint to provide higher power in the same space. Additional features include up to 94% efficiency, rated operating temperatures extending from -40C to +70°C, parallel power up to 4,000W, and compliance with ANSI/AAMI ES60601-1 and IEC 60601-1 medical safety requirements.

Our NMP Series modular, medical-grade power supplies are designed for systems that require multiple DC voltages and have limited space. They’re available with 5V, 12V, 24V, and 48V output modules that can output up to 240W and are suitable for use in 3–55V voltage applications, including dental chairs equipped with lifting motors, air compressors, resin curing lights, and water pumps that all require different voltages. Key product features include a modular design with flexible output configurability, built-in voltage and current adjustment functions, a low-profile, a slim 1U form factor, compliance with ITE (62368-1) and medical (60601-1) safety standards, a 2xMOPP isolation rating, and a five-year warranty. They can also be connected in parallel in applications with several high-current components, such as laser or image rendering equipment, including MRI machines.
Moving on to external power adapters, our GSM Series high-efficiency, medical-grade external power adapters is based on our proven GST Series adapters for consumer and industrial electronics and information technology and telecom equipment. They’re equipped with IEC320-C14 (Type A) and IEC320-C8 (Type B) AC input connections, can be modified to include different types of DC output connectors, and are rated for 5–48V. Key product features include DoE Level VI/ERP high efficiency, a 2xMOPP isolation and BF-type medical application rating, and a three-year warranty.
Since external power supplies like these are often used for powering portable medical devices and charging wearable medical devices used in household settings, they don’t always have to comply with medical standards like the GSM Series does. But compliant devices can satisfy the needs of a broader range of applications, including portable medical devices in clinic settings, and can help ease customer concerns about safety, which can be beneficial from a marketing perspective.


Our newest line of external power adapters, the NGE Series, builds on this concept, offering IEC safety certifications for the three different application areas they can be used in — medical electronics, consumer electronics, including information technology and telecom equipment, and household appliances — as well as general transformer, reactor, and power supply safety, which some countries still use in lieu of IEC 60601-1 for medical safety. NGE Series power adapters also come with CCC, BSMI, and PSE certifications for use in China, Taiwan, and Japan and are rated for operating temperatures extending from -30°C to +70°C to ensure compliance with various country-specific requirements. In addition, they feature interchangeable AC plugs that support international compatibility for both OEMs, who can now serve the global market with a single SKU, and the growing number of home healthcare patients who aren’t confined to their homes and may travel to places with different wall outlets.
To further support portability, the new NGE Series has a slim, 30mm profile design that prevents it from obstructing adjacent outlets and makes it easy to travel with. It’s also compliant with 2xMOPP and BF-type medical applications and designed to deliver efficient, high-quality performance over a long lifetime. RS currently has several of our 12W and 18W NGE Series adapters on order and will soon stock our 30W, 45W, 65W, and 90W models as well.
Is there anything else you’d like RS customers to know about MEAN WELL or its medical power supplies?
MEAN WELL offers the world’s most comprehensive selection of standard, off-the-shelf power supplies and combines breadth and depth with design-in ease and efficiency. Our product portfolio includes more than 13,000 SKUs designed for low- to high-power applications up to 30kW in all kinds of industries, already approved to a wide range of the global electronics industry’s most common general- and market-specific safety standards, and backed by more than 42 years of expert R&D and field-proven quality, performance, and reliability.
When Power is Paramount, Trust MEAN WELL and RS
MEAN WELL is a leading global supplier of standard power supply products renowned for their quality and performance and utilized in a wide variety of application areas, including the medical, energy, lighting, building automation and control, manufacturing, telecommunications, environmental monitoring, security monitoring, property management, and access control industries.
RS offers thousands of in-stock MEAN WELL power supplies, including several series of power supplies engineered to satisfy the stringent requirements of medical electronics applications. For more information about MEAN WELL and its extensive selection of standard power supplies, please visit the links embedded here. For assistance identifying, procuring, deploying, and maintaining MEAN WELL power supplies sure to satisfy your unique application demands, please contact your local RS representative at 1.866.433.5722 or reach out to the RS technical support team.